Science of the Comstock - Chemistry
Home | Tour | Physics | Chemistry | Earth Science | Environment | Scams | Lesson Plans
Chemistry Topics:
Introduction
Ore Processing
Fire Assaying
Ore Processing
Introduction
Metals are taken from the rocks of an ore deposit using chemistry. In processing ore to recover the metal of interest, steps of the process can be divided into (a) the steps to prepare the metal for separation from the rest of the rock or other metals, and (b) steps to actually remove the metal from the rock and unusable or uninteresting parts of the ore. The type of reaction used to extract the metal in both steps depends on the type of ore. That is, the chemical reaction to produce the metal depends on the way the metal is combined with other elements in the rock. The reactions used also change with time as new, better methods for extracting the metal from the rock to put it in a form usable to the consumer are developed.
In Virginia City ores, the silver was most often combined with sulfur to form a sulfide: Ag2S. However, it also was found combined with other elements. The method of preparing the metal of interest for removal from the rock had to be able to break up the sulfide compound as well as other compounds that might include the metal of interest.
The earliest method of extraction was a method developed in the Mexican mines in the mid 1500s and familiar to the miners at Virginia City, the Patio Process. This method of processing the rocks to extract the silver was slow, did not recover all the silver, and did not use the added reagents as efficiently as possible. In addition, the climate of Virginia City caused problems with freezing.
As the metal was ground in circular open patios or "arrastras," water, mercury, salt and roasted copper and iron sulfides were added. (reference: Thompson, James V., "Silver Recovery by Older Methods," Engineering and Mining Journal, June 1991, p. 39-41). Although the chemicals were added all at once, it is easier to think of the reactions in sequence. The first several reactions change the silver compounds into silver metal (step (a)), then the silver metal is removed from the waste rock and other metals by amalgamation (step (b)).
Production of Silver Metal Using the Patio Process
Preparing the silver for removal (step (a): The roasted copper and iron sulfides turn into copper sulfates (CuSO4) and iron sulfates (FeSO4). The salt (NaCl) reacts with the silver sulfide (Ag2S) in the open air and light to form silver chloride (AgCl):
Ag2S + 2NaCl + 2O2 = Na2SO4 + 2AgCl
Then, the silver chloride (AgCl) reacts with the mercury (Hg) to form silver metal.
2AgCl + 2Hg = 2Ag + Hg2Cl2
This process was carried out in the light and open air. The open air provided the oxygen (O2) for the conversion of sulfide to sulfate (SO4-2). The light may have also helped. The sunlight may have provided some heat (Smith, Grant H., The History of the Comstock Lode, Nevada Bureau of Mines and Geology, Nevada Press, Reno, NV, 1966 and 1998, p. 41) to help the reaction that produced the silver chloride. In addition, the action of light on silver halides serves to reduce the silver to the metal as in the photographic process in which metallic silver and chlorine gas are produced from silver chloride..
2AgCl + light = 2Ag + Cl2 (applies to other silver halides, too)
Separating the Metal from the Waste Rock
Removing the silver metal from the waste rock (step (b)): The silver metal forms an amalgamation with excess mercury:
Ag + Hg = HgAg (amalgam)
In order to recover the silver metal from the amalgam, the amalgam is heated in a "retort" until the mercury vaporizes and leaves behind the silver metal. The mercury from this step of the process can be recovered to be used in another extraction.
Improvement of the Patio Process
Through several reactions, the cupric sulfate reacts with the sulfur to remove it from reacting with the silver again:
CuSO4 + 2 NaCl = CuCl2 + Na2SO4
2CuCl2 + 2Hg = 2 CuCl + Hg2Cl2 (production of monovalent copper)
Ag2S + 2CuCl = 2 AgCl + Cu2S (Copper forms copper sulfide and removes the sulfide from the reaction.)
AgCl + CuCl = Ag + CuCl2
The silver metal released by reaction with cuprous chloride is then amalgamated by the mercury. The addition of cupric sulfate encourages the reduction of silver because the drive (electromotive force) favors the reduction of silver chloride by cuprous chloride (last reaction) is nearly 0.3 volts greater than the reduction of silver chloride by mercury (at standard conditions of one atomsphere pressure, one molar solutions, and room temperature). The silver so produced still needs to be removed from the rest of the rock, by amalgamation in this instance.
The Washoe Process
In the Washoe Process (named for Indians that inhabited the area around Lake Tahoe, and thus the name for the area around Virginia City, the name now preserved in the name of the neighboring county, Washoe County), the improvement to the process of heating the ore during extraction in an iron pan increased the recovery and decreased the processing time. The iron from the pan acted as the reducing agent for the silver:
2AgCl + Fe = 2Ag + FeCl2
The drive for this reaction is nearly 0.6 volts greater than the drive for the reaction for reducing silver using copper (at standard conditions as above), so this reaction is highly favored.
In addition, heating the reaction mixture helped the formation of the amalgam of silver with mercury. In this reaction, the mercury was not changed into mercurous chloride (calomel), so mercury was not used up in the process. The iron pans and iron mixers (mullers) would be consumed in the process, but these could be replaced readily.
The left photo is of the interior of a mill on the Comstock using the Washoe Process. The right photo shows the exterior of the Brunswick Mill on the Carson River.
(Reference: Dennis, W.H., A Hundred Years of Metallurgy, Gerals Duckworth & Co., Ltd., London, 1963, p. 282-287)
Activity: Metal from Rocks
Elisabeth M. Price, Washoe County School District, Reno, Nevada
Dana Sue Kimbal, Newmont Mining Corporation
Introduction
This activity models a modern method for the extraction of gold and other precious metals such as silver from rocks.
The steps of the Washoe Process modeled here include the grinding and preparation of ore for extraction with mercury, and the reduction of the precious metal so it can be processed into the pure metal. The former step is the same as the modern preparation of the heap for leaching. In the Washoe Process, mercury was used to extract the metal from the rock. Mercury is too dangerous to use industrially or in the classroom, so in modern methods, cyanide is used for the extraction; however, in this model, extraction is carried out by simple water. The latter step of reducing the metal for processing is represented by the precipitation of the copper onto the iron paper clip. This step also models the reduction of the silver by the iron extraction pan in the Washoe Process.
Key Concept
Copper is leached from finely ground rock and recovered by reduction on iron paper clips. The activity models a heap leach pad and the modern procedures for recovering some metals, particularly gold and copper.
SKILLS: Observing, Recording, Investigating, and Modeling
TIME: 50 minutes
AUDIENCE: Teachers and students, grades 5 - 8.
Objective
To understand the structure of a heap leach pad and the chemistry involved in leaching and recovering the metal from solution by making a model using copper as the recovered metal.
Safety
Wear chemical splash goggles.
Background: Content Focus
Some compounds of metals are soluble in various solutions. These metals can be dissolved from a finely ground rock, and the solution with the metal compound can be recovered for further processing.
Here, cupric sulfate pentahydrate (CuSO4. 5H2O) is mixed with finely ground rock or dirt. Cupric sulfate is soluble in water, so this is leached from the rock mixture with water. The water is collected in a plate for further study. Interferences can include other compounds in the dirt mixture which are also water-soluble.
This step depends on the relative solubilities of the cupric sulfate and rock in water. If cupric sulfate were not soluble in water, different solution would have to be used to leach the cupric sulfate from the rock mixture.
The copper is separated from the solution by precipitating the copper metal on an iron paper clip. (Reduction of the cupric ion in solution to copper metal and oxidation of the iron paper clip to ferrous sulfate in solution.) The reaction can be written:
CuSO4 (aq) + Fe (s) ® Cu (s) + FeSO4 (aq), or
Cu+2(aq) + Fe (s) ® Cu (s) + Fe+2(aq)
The paper clips need to be only iron, not nickel coated. Steel wool can be used also.
Not only does the paper clip become the characteristic copper color, but also the blue color of the copper ion in solution disappears from the solution, indicating that the copper ion (Cu+2) has been removed from solution.
Background Related to Mining
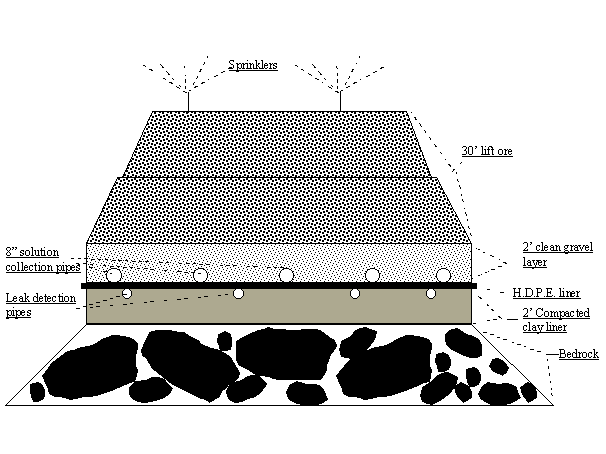
This picture shows the heap and pregnant solution pond at Newmont Mining Corporation's Lone Tree Mine.
Leach Pad
The structure of the leach pad actually used in mining is designed for efficient leaching and environmental safety. In a mine, the construction of the leach pad begins with placement on the bottom of about 12 inches of a non-permeable layer of clay. On top of that is a system of pipes to detect any possible leaks in the pad. This is covered with a plastic sheet (80 mil). On top of the sheet is the system of pipes with holes to collect the solution as it comes through pile of rocks. A porous layer of rock covers this. Then, the ore is heaped on top. The leaching solution is introduced to the heap with a sprinkler system.
Recovery of the metal
The solution with the dissolved metal from the ore is collected and sent to a processing plant. The metal in the solution may be concentrated in one step, but eventually the metal ions in solution are reduced to the metal either by precipitation onto a more reactive metal as in our model, or by "electrowinning," a process in which an electric current passes through the solution and the metal deposits on the cathode of the electric cell.
The solution that no longer has any metal in it can be reused for more leaching on the heap.
Reclamation of the leach heap
Everything in mining must be returned to a condition as nearly natural as possible. This means the leach pad must be made environmentally benign by rinsing or bioprocessing so the water that flows off of the heap meets water quality standards. The slopes of the heap must be stabilized, and the hill must be planted with grasses and shrubs.
Gold Recovery
Although gold leaching is not as visible as copper leaching, the most common method of leaching gold uses cyanide, oxygen and water:
4Au + 8NaCN + O2 + 2H2O ® 4NaAu(CN)2 + 4NaOH
Copper Recovery
Some copper ores are processes with heap leaching. Sulfuric acid is used to dissolve copper carbonate ores. The general reaction is
Cu2CO3(OH)2 (s) + 2H2SO4 ® 2CuSO4 + H2CO3 (aq) + 2H2O
Copper sulfate is soluble in water, so can be readily processed. The carbonic acid (H2CO3) will probably break down to carbonate and water: H2CO3 ® H2O + CO2.
Advance Preparation and Tips
Advance Preparation
Prepare the finely ground rock either by grinding some rock in a ball mill, or by sieving mixed rocks, or using sand. Mix in powdered cupric sulfate pentahydrate to form a 1% mixture with the rock (1g CuSO4. 5H2O plus 99 g rock.)
Tips
Nickel coated paper clips do not react as readily as uncoated iron paper clips with the copper in the copper solution, so use non-coated paper clips or steel wool.
The precipitation of the copper metal generally produces an orange precipitate on the iron paper clip. The precipitate starts out black, and gradually takes on an orange color as more copper precipitates. Occasionally, nice copper crystals form. This normal orange color can be mistaken for rust (hydrated iron (III) oxide). Observations can show that this is not the material formed. A control in which a paper clip is dropped into plain water does not produce the orange precipitate as does the experimental situation in which copper is present in solution. Notice that the blue color of the copper sulfate solution disappears as the amount of orange precipitate on the paper clip increases. This means the copper is being removed from solution.
Washing the model heap for reclamation may take prohibitively long. If so, continue with a discussion of how the situation might be improved at an actual mine. First, the ore would probably be nearly completely recovered, so washing to remove residual ore metal would probably not be so important. Trace metals might be a problem and require some special treatment to immobilize or remove those metals. Bacteria play an important part in preparing the heaps for reclamation because bacteria have been used specifically to remove problem metals from the system.
Materials
For each student:
Styrofoam bowl with holes poked in the bottom
(Alternately, insert short coffee stirrer straws into low holes to simulate the pipes that carry the gold bearing solution)
Coffee filter
Styrofoam plate
Finely ground rock or sand
Cupric sulfate pentahydrate, powder (CuSO4 . 5H2O)
Squirt bottle for water leaching solution
Iron paper clips
Clear plastic cup (9 oz) or top of Styrofoam "clam shell" sandwich container for processing tank
Procedure
- Prepare the leach pad by punching holes in the Styrofoam bowl. Punch holes up the side on one side so the solution can drip out of the side of the tilted apparatus. The bowl with holes in represents the pipes with holes in that lead the solution with the dissolved metal to the collection troughs. (Alternatively, use a round tooth pick to punch 4 holes at the bottom of half a small Styrofoam "clam shell" sandwich container. Put 1.5" pieces of coffee stirrer straws into the holes to simulate the pipes that carry the gold bearing solution.)
- Place filter paper in bowl to keep ore rock in the bowl. The filter paper represents the rock that covers the perforated collection pipes.
- Build the leach heap by putting the mixture of crushed rock and cupric sulfate into the bowl lined with the coffee filter.
- Place the bowl with the crushed rock on a Styrofoam plate. This plate represents the plastic liner at the bottom of the leach heap. The impermeable clay layer is represented by the worktable.
- Tilt the whole apparatus by lifting one side up on an overturned bowl.
- Leach the heap by squirting water gently all over the heap until at least 10 mL of water with dissolved cupric sulfate have collected at the bottom of the Styrofoam plate.
- Take the solution (pregnant solution) to the processing plant by using a pipette to withdraw about 10 mL from the bottom of the leach heap plate. Place the 10 mL in a clear 9 oz cup.
- Recover the copper from solution by adding a paper clip. Record the observations after the paper clip is added to the solution.
Extensions
Study reclamation of a heap leach pad by removing the soluble metals from the pile through repeated rinsing. (How is it possible to tell that the pile is clean? Analyze for chemicals that are limited by drinking water standards. In this case, check for copper in solution by adding ammonium hydroxide. The dark blue ammonium-copper complex extends the visible detection limit of copper.)
Prepare the heap leach pad for reclaimed use. (What else must be considered? Slope stability, soil preservation methods)
Suggested Follow-up Discussion
What problems were encountered with the leaching? The fines in the "ore" sample clogged the filter. The solution did not go all the way through the heap. Some may not have gotten wet.
How could those problems be overcome? The fines could be eliminated through agglomeration. Solutions could be added to the leach so the rocks got completely wet. The angle of the heap pad could be changed and the spraying could be placed carefully so the solution has to go through the whole leach heap.


 Home
Home